New study provides a biological basis for drug hypersensitivity in adolescents and some adults with a genetic variant
In my first “primary article briefs” blog post, I am covering two companion articles published in the March 1st, 2016 issue of the open-access journal, eLife, by Mauro Costa-Mattioli’s group at Baylor College of Medicine and colleagues (Huang, et al., 2016; Placzek, et al., 2016).For the first time, the authors describe a biological explanation for why adolescents have a higher susceptibility to the effects of drugs and drug addiction than (most) adults. They also sh ow that this same biological mechanism can be used to understand why some people (who express a particular gene variant) are more susceptible to drug addiction than others. This finding not only opens the door to the possibility of designing pharmaceutical agents that could help reduce the effects of drugs on adolescents and others who have this gene variant, but it more importantly helps us to frame the argument that drug addiction is less of a behavioral problem and more of a biological/medical problem that needs to be dealt with in a medical manner and not by shaming and shunning the addict.
ow that this same biological mechanism can be used to understand why some people (who express a particular gene variant) are more susceptible to drug addiction than others. This finding not only opens the door to the possibility of designing pharmaceutical agents that could help reduce the effects of drugs on adolescents and others who have this gene variant, but it more importantly helps us to frame the argument that drug addiction is less of a behavioral problem and more of a biological/medical problem that needs to be dealt with in a medical manner and not by shaming and shunning the addict.
To understand what the researchers did and why, it is important to understand the basic biology of drugs and addiction. Recreational drugs like cocaine, alcohol, and even nicotine produce their pleasurable (and subsequent addictive) behavior by activating the “reward” centers of the brain, located in the ventral tegmental area (VTA) in the midbrain and the nucleus accumbens (NAc) of the basal forebrain. Excitatory, glutamatergic neurons (neurons that secrete the general excitatory neurotransmitter, glutamate) signal into the VTA to activate projection neurons to the NAc that secrete dopamine (DA), which is the primary neuromodulatory neurotransmitter tha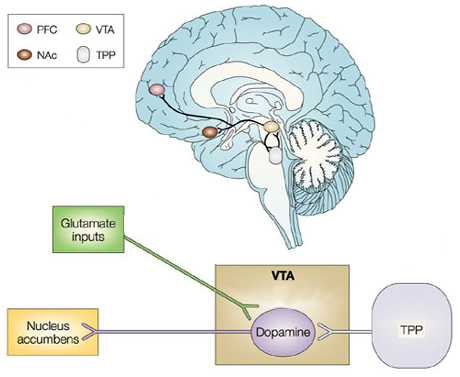 t regulates the limbic basal ganglia and its functions. The limbic loop of the basal ganglia is the emotional center that produces feelings of pleasure, love, motivation, and reward. All addictive drugs produce their pleasurable and drug-seeking effects by impinging somewhere along these dopaminergic neural signaling networks.
t regulates the limbic basal ganglia and its functions. The limbic loop of the basal ganglia is the emotional center that produces feelings of pleasure, love, motivation, and reward. All addictive drugs produce their pleasurable and drug-seeking effects by impinging somewhere along these dopaminergic neural signaling networks.
The two companion articles that I am writing about show that it only takes a small concentration of drug (whether it be cocaine or nicotine) in adolescent mice to strengthen the synapses that activate the dopaminergic (DA) neurons in the VTA that project to the NAc. It takes a much higher concentration of drug, however, to do the same thing in adult mice. They also show that this effect of synaptic strengthening in the VTA correlates with drug-seeking behavior, and that it takes more drug to cause this drug-seeking behavior in adult mice as compared to adolescent mice. This finding is not new, but lays out the basis for their subsequent experiments to explain why adolescents have a lower threshold at which recreational drugs can elicit their addictive effects.
Previous studies have shown how changes in gene transcription (either through the activity of transcription factors or epigenetic mechanisms) contribute to drug addiction-related behaviors, but these studies did not provide an explanation for the differences in susceptibility to drugs between adolescents and adults. To investigate this difference, Mauro Costa-Mattioli and colleagues made two observations that led them to focus on the regulation of protein synthesis: 1) protein synthesis is required for cocaine-induced synaptic strengthening in the dopaminergic neurons of the VTA and subsequent drug-seeking behaviors, and 2) protein synthesis is reduced in the brains of adult mice as compared to the brains of adolescent mice. Therefore, they hypothesized that a difference in regulation of protein synthesis between adolescents and adults explains the difference in their susceptibilities to drugs and drug-related behaviors.
Through a series of elegant experiments, the authors demonstrate a difference between adolescents and adults in the level of drug-induced activation of a specific protein that blocks protein synthesis/translation. Specifically, the drug-induced activation of eIF2alpha (indicated as p-eIF2alpha, a phosphorylated form of eIF2alpha) is diminished in adolescents as compared to adults. The result of this reduced p-eIF2alpha is an increase in general translation (meaning that more proteins are made), thus allowing for increased synaptic strength (or long-term potentiation [LTP]), and a decrease in translation of a specific set of proteins that are involved in decreasing synaptic strength (or long-term depression [LTD]). The authors show that the difference in drug-induced synaptic strengthening (LTP) and drug-seeking behaviors between adolescents and adults is due to this difference in activation of p-eIF2alpha (Huang, et al., 2016).
The brains of children and adolescents are more “plastic” (meaning that they change more) than the brains of adults because they are still growing and forming proper connections. Therefore, it is not surprising that adolescents would be more susceptible to drug-induced synaptic strengthening, a type of neuroplasticity, than adults. What is surprising, however, is the neural specificity of this finding – that only DA neurons in the VTA, and not in the NAc, show this drug-induced synaptic strengthening. The authors do not delve into the normal biology that could explain this difference between adolescents and adults; to do that they could compare the dose-dependent effects of the cognate neurotransmitter (glutamate) on synaptic strengthening of these DA neurons in adults and adolescents. It would be interesting to see if glutamate (the cognate neurotransmitter for these synapses) shows a similar differential effect on synaptic strengthening as the drugs tested in these studies, or if this differential effect is specific to these drugs. If the effect is similar, then it would suggest a biological, developmental function for the increased neuroplasticity of the DA neurons in the VTA of adolescents – suggesting that adolescence is a particularly important period of life when our motivations are most strongly created in the reward system of our brain. Certainly, this seems to be true on an intuitive level.
The primary new finding of the second paper (Placzek, et al., 2016) is a variation in the gene for eIF2alpha (the gene name is Eif2s1) that increases the vulnerability of human smokers to addiction. Essentially, the authors show that, similar to adolescents, adults with this genetic variation in Eif2s1 have an increased susceptibility to addiction-associated phenomena. One hallmark of addiction is that addicts develop a dampened response of the “emotional reinforcement circuitry” to less potent natural rewards, while their response to addictive drugs intensifies (Neuroscience, 5th ed., 2012). Using juice as a natural reward, the authors show that tobacco smokers with t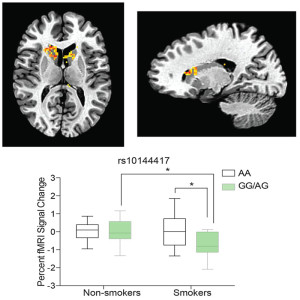 he Eif2s1 gene variant had reduced activity in their reward center (shown by functional magentic resonance imaging [fMRI]) compared to smokers without the genetic variation or non-smokers (shown in the figure to the left – the variation is a single nucleotide variation [SNP] changing an A to a G in the regulatory sequences; above is a transverse (L) and a sagittal (R) view showing the region in the brain (NAc) where the activity is reduced; below is quantification of the fMRI signal change). These results indicate that people with the genetic variation in Eif2s1 have an increased risk for drug addiction than those without the genetic variation.
he Eif2s1 gene variant had reduced activity in their reward center (shown by functional magentic resonance imaging [fMRI]) compared to smokers without the genetic variation or non-smokers (shown in the figure to the left – the variation is a single nucleotide variation [SNP] changing an A to a G in the regulatory sequences; above is a transverse (L) and a sagittal (R) view showing the region in the brain (NAc) where the activity is reduced; below is quantification of the fMRI signal change). These results indicate that people with the genetic variation in Eif2s1 have an increased risk for drug addiction than those without the genetic variation.
The authors further show that the variant SNP in the Eif2s1 gene causes increased protein levels of eIF2alpha, which results in decreased activation (p-eIF2alpha) in response to drugs. The authors do not show the mechanism by which the increased levels of the protein lead to decreased activation, but provide some hypotheses (e.g., stoichiometric imbalances, sequestration, or compensatory feedback mechanisms in other translational machinery proteins). They also discuss the need to further investigate the role of p-eIF2alpha in later stages of addiction, since these studies were focused on the initial exposure to drugs. Furthermore, they believe that p-eIF2alpha may be a promising new target for the medical treatment of addiction.
My take-home message from these papers is that we now have a specific biological marker for susceptibility to drug addiction. This finding not only provides more evidence of the biological/medical basis of addiction (rather than just a behavioral disorder), but it also provides a promising new target for medical intervention. Ultimately, this research supports the notion that it is important to treat drug addiction as a medical disease rather than a crime or misbehavior.




Recent Comments